Introduction
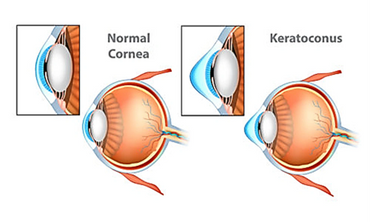
Corneal cross-linking (CXL) is a procedure that uses UV light to strengthen the cornea. The goal of the treatment is to halt progressive and irregular changes in corneal shape known as ectasia. These ectatic changes are typically characterized by corneal thinning and an increase in the anterior and/or posterior curvatures of the cornea, often leading to high levels of myopia and astigmatism. The most common form of ectasia is keratoconus, and it is less commonly seen after laser vision correction, such as Lasik.
Cross Linking Background
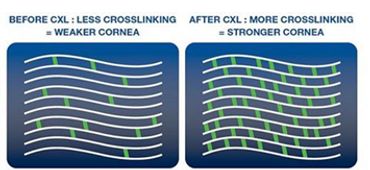
Collagen cross-linking refers to the ability of collagen fibrils to form strong chemical bonds, known as cross-links, with adjacent fibrils. In the cornea, collagen cross-linking occurs naturally with aging due to an oxidative deamination reaction that takes place within the end chains of collagen. It has been hypothesized that this natural cross-linkage of collagen explains why keratoectasia (corneal ectasia) often progresses most rapidly in adolescence or early adulthood but tends to stabilize in patients after middle age.
The foundations for corneal collagen cross-linking procedures were developed in Europe by researchers at the University of Dresden in the late 1990s, with clinical trials ongoing since the same year. UV light was used to induce collagen cross-linking in soaked porcine and rabbit corneas through the oxidation pathway. The resultant corneas were shown to be stiffer and more resistant to enzymatic digestion. Investigation also demonstrated that the treated corneas contained higher molecular weight polymers of collagen due to fibril cross-linking. Safety studies revealed that the endothelium was not damaged by the treatment when proper UV irradiance was maintained and if the corneal thickness exceeded 400 microns.
Corneal collagen cross-linking is FDA approved in the United States.